Once intrathecal drug delivery has been selected as an
appropriate therapy for a patient with chronic pain, the patient
must go through a screening test to help predict the level of
response to the therapy. The intrathecal drug delivery screening
test has two stages:
● Delivery of a pain-relieving drug such as morphine to the
patientís intraspinal space
● Longer-term patient evaluation
 The screening test
The screening test
The delivery of a pain-relieving drug to the patientís epidural
or intrathecal space is the first stage of the intrathecal drug
delivery screening test, and often takes place on an outpatient
basis. Physicians may elect to gradually withdraw or decrease a
patientís systemic opioids before the screening test to reduce
the overall steroid burden and to allow a more accurate reading.
However, patients may continue to take certain medications,
including oral morphine during the screening test, if the
physician deems it necessary. The physician will determine the
protocol for the screening test.
 Possible protocols
Possible protocols
The exact screening protocol is derived at the discretion of the
physician. However, screening trial techniques can generally be
categorized as follows:
● Single bolus injection
● Multiple injections
● Continuous infusion
|
Table 1: Screening trial
techniques |
|
Method |
Description |
| Single bolus injection |
● The patient is injected
with a single bolus of a pain-relieving drug into
the intrathecal space via a lumbar puncture
● The dose is usually 0.5 to 1.0 mg or the
intrathecal equivalent of the patientís daily
(systemic) narcotic intake |
| Multiple injections |
● The patient is
administered a series of injections, either
intrathecally or epidurally
● For epidural administration, injections are
administered via an epidural catheter inserted under
fluoroscopy to ensure proper placement
● Patients may receive a placebo to accurately
assess symptom relief |
| Continuous infusion |
● A catheter is placed
either intrathecally or epidurally and connected to
an external infusion pump
● The effectiveness and tolerability of the drug is
tested over a period of days to weeks. The initial
dose is 0.2 mg/hour or the epidural equivalent of
the patientís daily (systemic) narcotic intake. The
dose is increased every 12 to 14 hours until pain
relief is reported
● The advantage of this method is that continuous
infusion can more closely mimic an implantable
system, and response can be assessed during the
patientís normal daily activities |
Physicians may choose one of several variations of the above
protocol. For example, physicians may choose to administer the
bolus injection into the patientís epidural space rather than
the intrathecal space. The physician may also choose to repeat
the bolus injection method by repeating the injection every 8Ė12
hours, increasing the daily dose, until adequate pain relief is
achieved. Variations of the protocol impact on the duration of
the screening test, which can be accomplished within 23 hours
using a bolus injection or within a few days or weeks using
continuous infusion.
During the screening test the physician will observe the patient
for the following:
● Treatment efficacy
● Treatment tolerability
Adverse events such as allergic or sensitivity reaction to the
drug are not usually life-threatening and most can be
effectively managed. However, adverse events may be the first
signs of potential overdose, which is particularly serious and
may lead to death without proper intervention.
Morphine overdose is characterized by respiratory depression or
arrest with or without central nervous system depression.
Symptoms of central nervous system depression include:
● Dizziness
● Sedation
● Euphoria
● Anxiety
Pupil dilation and/or seizures may also occur during morphine
overdose
 Patient evaluation
Patient evaluation
Patient evaluation is the second stage of an intrathecal drug
delivery screening test. Patient evaluation usually involves
both the patientís self-evaluation and the clinicianís
assessment of the patientís pain relief. Patient evaluation
takes place simultaneously with the screening test when the
bolus injection protocol is used. However, although patient
evaluation with the continuous-infusion protocol often takes
place simultaneously with the screening test, it can also occur
later during a patients normal everyday activities. In this
case, patients are encouraged to keep a detailed journal of
their pain during the time that they are away from the
physician. This journal helps to provide an accurate patient
evaluation and assists in programming the implanted pump. As
determining a patientís pain relief can be highly subjective,
the physician will determine whether the patient has a
clinically significant response which is generally considered to
be a 50% or greater reduction in pain.
Possible evaluation strategies include:
● Comparing pain scale information before and after the
screening test
● A verbal assessment of a patientís perception of effectiveness
● Looking for signs of increased physical activity
● Reviewing the patients pain journal (if a continuous infusion
protocol was used)
Some physicians work with physical therapists during the
screening test to evaluate functional improvement in the
patients indicative of a clinically significant reduction in
pain. In general, if a patient reports at least a 50% reduction
in pain with tolerable side effects, it is considered a positive
response and the patient
and the physician may decide to proceed with the implantation of
a intrathecal drug delivery system. If the screening test was
not successful a complete system should not be implanted.
 Complete system implant
Complete system implant
Complete system implant is undertaken after a positive screening
test. Generally, the physician will check for infection in the
patientís body 1Ė2 days before implant. The implant procedure
may be delayed if infection is found. The intrathecal drug
delivery system is implanted using a sterile surgical procedure
performed under local, regional or general anesthesia. The
implantation procedure typically lasts between 1 and 2 hours.
The pump is generally implanted subcutaneously in the right or
left abdomen where there is sufficient skin and subcutaneous
tissue to support the implanted system.
The following implantation procedure is for SynchroMedģ EL only.
 Preparing the patient
Preparing the patient
The first step in the implantation procedure is to prepare the
patient. The details of this are shown in the table below.
|
Table 2: Preparation of the patient |
| 1. |
Complete preoperative physical examination and
patient education |
| 2. |
Select appropriate site for the pump on the
patientís abdomen before positioning |
| 3. |
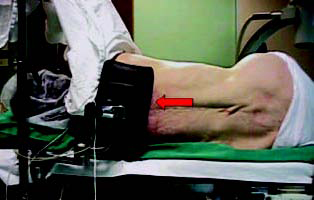
Positioning of the patient on the operating table Ė
preferably in a lateral recumbent position |
| |
 |
| |
Figure 1 |
| 4. |
Drape the patient, exposing both the pump site and
catheterization site. This allows for pump and catheter
implantation without additional draping |
 Preparing the pump
Preparing the pump
Preparation of the pump, especially the purge programming and
empty/refill procedures should be completed only when
intraspinal access has been achieved with the spinal catheter.
Stages in pump preparation are shown in the table below.
|
Table 3: Preparation of the pump |
| 1. |
The initial pump status (the reservoir volume etc) should be
checked while the
pump is still in the sterile package |
| 2. |
The pump should be pre-warmed for 15Ė20 minutes at 35Ė400C
(or 95Ė1050F)
by placing the pump in the blanket warming cupboard |
| 3. |
The sterile package should be opened and the pump removed.
The pump should
be placed in a basin of warm water or saline (35Ė400C) until
purge is complete |
| 4. |
When purge is complete, the warm pump should be emptied using
a 20 ml
syringe and a 22-gauge huber-type needle. The pump should be
kept in a warm
saline bath during this procedure keeping the water temperature
around 35Ė400C |
| 5. |
The pump should be filled with the appropriate amount of
prescribed drug using
the same 22-gauge huber-type needle |
| 6. |
If there is no suture loop, the pump should be placed in a
mesh pouch where it
is now ready for implantation |
 Implantation of the catheter and pump
Implantation of the catheter and pump
The next step in the implantation process is to implant the
catheter and the
pump. The physician will carry out the complete system implant
procedure
by:
● Inserting the 15-gauge Tuohy needle into the lumbar region
● Inserting the catheter through the needle and threading the
distal tip of the catheter to the desired location.
● Verifying catheter position using fluoroscopy.
● Making a small vertical incision alongside the Tuohy needle to
expose
the supraspinous ligament or deep fascia.
● Withdrawing the needle and guidewire
● Preparing the pump pocket by making an incision in the lower
abdomen, 2.5 cm beneath the skin.
● Making a subcutaneous tunnel between the spinal incision site
to the
pump pocket with the tunneling tool
● Tunneling the catheter to the pump pocket and connecting it to
the pump.
● Trimming and anchoring the catheter securely.
● Placing the pump in the prepared pocket and closing all the
incisions.
After the system is implanted, the patient is
transported to the recovery
room, where the physician will program the pump. Programming
allows the
physician to enter the exact protocol for drug delivery and
helps to prevent
accidental overdose. The pump can then be programmed by the
physician
as needed during follow-up visits.
 Postoperative management
Postoperative management
Once the pump is implanted, the patient is closely monitored.
Postoperative care involves the following:
● Management of complications
● Continued patient education
● Dosage adjustment
 Surgical complications
Surgical complications
Patients who have poor nutritional status, are small in build
and/or thin, or
who have generally poor health are at greater risk for
post-surgical
infections. Potential surgical complications include:
● Infection
● Spinal headache
● CSF hygroma (an accumulation of cerebrospinal fluid which
produces
visible swelling)
● CSF leakage around the catheter insertion site
● Bleeding
● Pain and discomfort
● Pump pocket seroma (an accumulation of fluid in the pump
pocket
and/or seroma spinal site)
The management of these surgical complications is shown in the
table below.
|
Table 4: Management of post-surgical complications |
| Post-surgical
issues/problems |
Patient management |
| Wound care |
● Patients should change the sterile dressings on the incision
site as
instructed by their physician
● Patients should comply with hospital policies and procedures
regarding would care and infection control |
| Infections |
● Patients should be aware of signs of infection e.g., redness,
pain
and swelling
● Infections should be treated aggressively with systemic
antibiotics
● Infections usually disappear with treatment and rarely
necessitate
removal of the system
● The pump should be removed promptly if meningeal infection is
present or if the reservoir becomes contaminated |
| CSF leakage |
● Patients should be vigilant for CSF leakage at the incision
site along
the catheter and in the pocket site
● Surgical resuturing or revision may be necessary |
| Spinal headaches |
● Spinal headaches may be caused by CSF leakage around the
catheter, or loss of CSF during the implantation procedure
● Oral analgesics can help to reduce pain
● Having the patient lie flat for 1 to 2 days following
implantation can
greatly reduce the risk of headache
|
| Spinal hygromas
(an accumulation of
cerebropsinal fluid
which produces
visible swelling) |
● These are typically found under the skin in the lumbar region
of the
back and result from leakage after intraspinal catheter
implantation
● They are typically small and self-limiting and disappear on
their own
without treatment
● An abdominal binder with pressure dressing at the spinal site
may help
● Hygromas may be aspirated, though the risk of infection should
first
be evaluated
|
| Bleeding |
● For patients at high risk of postsurgical bleeding, standard
medical
practice for postoperative management of patients on
anticoagulant
therapy should be followed
● Epidural hematomas (swelling from blood accumulation caused by
blood vessel breaks) are rare but could produce severe
complications
● Patients should be vigilant for signs of epidural hematomas
such as
severe back pain, sudden onset of leg weakness and spasms, loss
of
reflexes in distal extremities and loss of bladder/bowel control
● Immediate treatment will be required |
| Incision pain and
discomfort |
● Pain and discomfort around the incision site and catheter are
common following surgery
● The duration and intensity of pain varies by individual
● Some patients may require ice at tunneling sites
● Mild analgesics e.g. acetaminophen and codeine can be
effective |
| Seromas (an
accumulation of fluid
in the pump pocket
and/or seroma spinal
site) |
● Seromas may be evident by the first postoperative day
● Signs and symptoms include swelling over the pump site and a
feeling of tightness over the pump site
● Pocket seromas often resolve without treatment but may persist
for
days or even weeks
● If persistent they can be aspirated, though the risk of
infection must
first be evaluated |
 System complications
System complications
Complications with the infusion system rarely occur but may
include:
● Catheter kink
● Catheter obstruction
● Catheter dislodgement, disconnection or breaks
● Programming errors
● Pump failure
System complications may present as drug overdose, loss of drug
effect or
withdrawal symptoms.
 Other complications
Other complications
In rare instances, the development of an inflammatory mass at
the top of
the implanted catheter may occur that can result in progressive
clinical
signs that bear monitoring. These signs include a progressive
change in the
character, quality or intensity of pain, an increase in the
level and degree of
pain despite dose escalation and sensory changes e.g., numbness,
tingling,
burning and hyperesthesia. Presentations that require immediate
diagnosis
include bowel and/or bladder dysfunction, myelopathy, gait
disturbances or
difficulty walking and paralysis. If the presence of an
inflammatory mass is
suspected, evaluation should include a review of the patient
history and
neurological evaluation, radiological diagnostic procedures e.g.
MRI and an
appropriate clinical consultation.
 Adverse drug events
Adverse drug events
Patients are monitored until the physician is confident that the
patientís
response to the drug is acceptable. Patients need to be aware of
the
potential averse effects of morphine so that they can
immediately
communicate any problems to their physician. The most common
adverse
effects reported with intrathecal morphine include:
● Pruritis (itching)
● Urinary retention
● Constipation
● Headache
● Peripheral edema (excessive fluid retention)
 Less frequent, but more serious adverse effects include:
Less frequent, but more serious adverse effects include:
● Respiratory depression
● Myoclonus
 Continued patient education
Continued patient education
Patients implanted with an intrathecal drug delivery system must
maintain
a long-term active involvement in their therapy and adhere to
lifestyle
limitations and precautions. Patients should be instructed to:
● Return for refills at the prescribed time.
● Consult their physician if they notice any unusual symptoms or
adverse events
● Notify other healthcare providers of the implanted pump and
the
medication it contains
● Consult their physician before scheduling any additional
therapies or
diagnostic tests (e.g., MRI, other drugs)
 Patients must also:
Patients must also:
● Avoid physical activities that may damage the implant site or
system
● Avoid extreme changes of altitude or pressure, which may alter
pump
flow rates
● Consult with a physician before enjoying saunas or steam baths
● Inform their physician about travel plans to avoid pump
emergency
refilling and maintenance
 Dosage adjustment
Dosage adjustment
The patientís initial drug dose is determined by monitoring his
or her
response to the screening trial. However, during the
postoperative period,
dosage adjustments may be necessary to achieve effective pain
management and minimize adverse effects. The upper daily dosage
limit for
each patient is assessed on an individual basis. Drug dosage is
typically
changed no more than once per 24 hours. |


 Whatís Up
Whatís Up